Pharmaconference 2018
- About Middle East Pharmacy and Pharmaceutical Conference
- Scientific Sessions of Pharma Conference 2018
- Market Analysis Report of Pharma Conference 2018
- Past Conference Report
- New updates: Pharmaceutical Sciences
PHARMA CONFERENCE 2018 CONGRESS
Middle East Pharmacy and Pharmaceutical Conference scheduled on September 24-25, 2018 Abu Dhabi , UAE. Conference includes Keynote presentations, Oral talks, Poster presentations, Symposia, Workshops, Exhibitions and Career development programs. The conference invites delegates from top Universities, Formulation Scientists, Researchers, Health care professionals, Pharmaceutical companies, students, business delegates and Young researchers, Health care professionals, Business delegates across the globe providing a better platform, interconnecting the latest research, technological developments as well as therapeutic aspects. Participating at Middle East Pharmacy and Pharmaceutical Conference will be good opportunity to meet eminent personalities in the fields of pharmaceutics and to learn the latest technological advancements.
Why to attend?
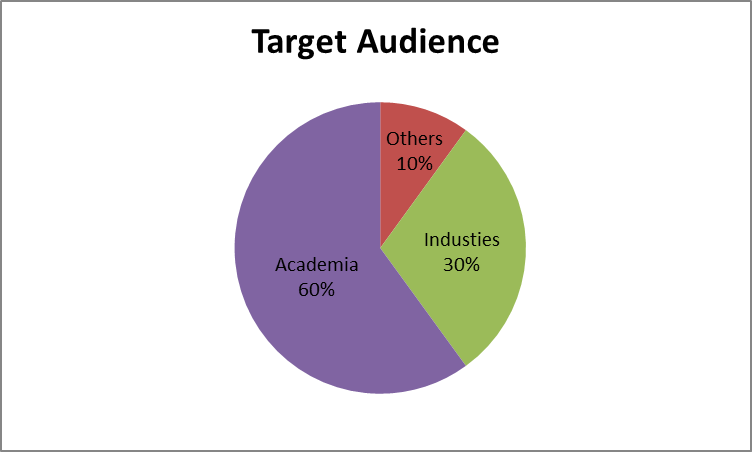
Pharma Conference 2018 is a platform to discuss the innovative researches and developments in the Pharmaceutical Science. World eminent speakers, the most recent research advances, and the latest updates in Pharma industry are hallmarks of this conference.
Target Audience:
Our Organization would be privileged to welcome the:
-
Pharmaceutical Researchers
-
Pharmaceutical Industry Professionals
-
Academicians
-
Pharmaceutical Associations and Societies
-
Post-Doctoral
-
Young Researchers and Students
-
Pharmacists
-
Pharmacy Technicians
-
Clinical trial experts in Pharmaceutical and Life science
-
Clinical scientists
-
Pharmacologists
Track 1: Drug Discovery & Development
Drug discovery and development together are the complete process of identifying a new drug and bringing it to market. Discovery may involve screening of chemical libraries, identification of the active ingredient from a natural remedy or design resulting from an understanding of the target. Development includes studies on microorganisms and animals, clinical trials and ultimately regulatory approval. The process of drug discovery involves a combination of many disciplines and interests starting from a simple process of identifying an active compound. The discovery of a new chemical entity that modifies a cell or tissue function is but the first step in the drug development process. Once shown to be effective and selective, a compound which is to be discovered must be completely free of toxicity, should have good bioavailability and marketable before it can be considered to be a therapeutic entity.
-
Drug target
-
Clinical Research
-
Computer-aided drug design
-
Ligand-based drug design
-
Genotoxicity studies
TRACK 2: Novel Drug Delivery System
Drug delivery is the process of administering the drug or pharmaceutical product, in order to achieve desired therapeutic effect. The method by which drug delivered is important, as it has significant effect on its efficacy. Novel drug delivery system(NDDS) involves various approaches like medical devices or drug-device combination products. NDDS involves combining pharmaceutics polymer science, and molecular biology. Novel drug delivery systems are designed based on physical and biochemical mechanisms. Physical mechanism or controlled drug delivery system includes dissolution, osmosis, erosion and diffusion. Biochemical mechanism includes gene therapy, liposomes, nanoparticles, monoclonal antibodies. NDDS we can make ideal drugs, controlled delivery, selective targeting at site of action. Various drug delivery and drug targeted systems are developed, in order to minimize drug degradation, drug adverse effect, and in order to increase the drug bioavailability (amount of drug available at site targeted region).
-
Hydrogel
-
Matrix Diffusion Types
-
Drug loaded erythrocytes
-
Osmotic Pressure Controlled
-
Chemically controlled
Track 3: Pharma and Technology
Nanotechnology is a study related to Nano sized particles, their function and practice with respect to different systems. The role of nanotechnology in the field of pharmaceutics has exceptionally altered the way of our understanding about drugs, Nano drugs or the use of nanoparticles as carrier of drug has become the basic fundamental or criteria for the production or design of a drug. Pharmaceutical nanotechnology is appliance of Nano science to pharmaceuticals as nanomaterials, and devices like diagnostic, drug delivery, biosensor, and imaging. Pharmaceutical nanotechnology has tuned diagnosis and focused treatment of disease at a molecular level. Nanotechnology is highly specialized field, which will revolutionize the pharmaceutical industry.
-
Nanomaterials
-
Drug carrier system
-
Molecular Diagnostics
-
Biosensor and biolabels
Track 4: Bioavailability & Bioequvialence
Bioavailability is defined as the rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action.Bioequivalence is defined as the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.
Bioavailability studies assess the relative fraction of the orally administered dose that is absorbed into the systemic circulation when compared to the bioavailability data for a solution, suspension, or intravenous dosage form and provide pharmacokinetic information related to distribution, elimination, effects of nutrients on absorption of the drug, dose proportionality, linearity in pharmacokinetics of the active moieties and appropriate inactive moieties.
-
First Pass Metabolism
-
Relative Bioavailability
-
Absolute Bioavailability
-
Therapeutic relevance
-
Pka and Pkd Models
Track 5: Pharmacogenomics
Pharmacogenomics (constitute both branch i.e. pharmacology and genetic) is the study of the role of genetics in drug response. It deals with the effect of genetic variation on drug response in patient by correlating the gene expression with the pharmacokinetic parameter i.e. absorption, distribution metabolism and excretions. The Pharmacogenomics is used in the research to increase the safety and efficacy of the drug by targeting the drug at the particular site of genes. Pharmacogenetics also play important role in the study of effect of multiple genes in the pharmacological action of drugs. Most of the drugs fail to show therapeutic effect at the latter stages it may be due to unexpected adverse effect of drug that may be due to the effect of various component of genes. Pharmacogenetics studies can be used at various stages of drug development. In clinical studies, pharmacogenetics can be used in stratification of patient based on their genotype, which correspond to their metabolizing capacity. This helps in prevention of various adverse drug reaction and better outcomes of clinical trial.
-
Toxicogenomics
-
Toxicogenomics
-
Pharmacoproteomics
-
Clinical Pharmacogenomics
-
Gene Polymorphism
Track 6: Pharmaceutical Formulations
Pharmaceutical formulation is a part of the drug development procedure. In this process Active ingredient is formulated into a final dosage form using proper excipients and technological procedures. A drug can have many forms depending upon how it is manufactured, route to be taken or the desired effects. The most common forms of drugs are tablets, capsules or solutions. Drug Formulation include Novel dosage forms, like tablets,sterile products, capsules, topical preparations, sustained-release and controlled release.
-
Topical and Transdermal Delivery
-
Ophthalmic Dosage Forms
-
Oral Solid Dosage Forms
-
Inhalation Dosage Forms
-
Parenteral Dosage Forms
Track 7: Clinical Research
Clinical trials are medical research studies involving people. They aim to test whether different treatments are safe and how well they work. Some trials involve healthy members of the public. Others involve patients who may be offered the option of taking part in a trial during their care and treatment. Clinical trials are carried out to try to answer specific questions about health and illness. Clinical trial is a systematic investigation in human subjects for evaluating the safety & efficacy of any new drug. Clinical trials are a set of tests in medical research and drug development that generate safety and efficacy data for health interventions in human beings.
-
Preclinical trails
-
Phases of clinical trails
-
Optimum dose finding
-
Single ascending dose limits
-
Multiple ascending dose limits
-
Post clinical trials
Track 8: Advances in Pharmaceutical Packaging
Packaging is the science, art and technology of enclosing or protecting products for distribution, sale and use. A final product is not accepted except it is properly Packaged and in some cases the major part of the formulation process may be concerned with selecting the correct package for the product. Packaging also refers to the process of Design, evaluation and fabrication of packages.
Advances in Packaging System: Blow-fill- seal technology is used to Produce aseptically sterile pharmaceuticals such as respiratory solutions, ophthalmics and wound care products.
Induction Cup sealing technology is used to create airtight and hermetic seals on the neck of the container based on the principle that a conductive material like aluminium foil heats up on exposure to high frequency magnetic field generated by an induction unit.
Tamper Evident Pharmaceutical Packaging have an indicator or barrier to entry which, if breached or missing, can reasonably be expected to provide visible or audible evidence to consumers that tampering has occurred.
Novel Laser Coding Technology enables glass containers for parenteral use to be clearly coded at the glass syringe manufacturer and tracked from production to end customer.
-
Film wrappers
-
Blister package
-
Strip package
-
Bubble pack
-
Shrink seal and bands
Track 9: Pharmaceutical Analysis
The Pharmaceutical analysis is a branch of chemistry, which deals with determination of the quality and quantity of the pharmaceutical products. It is used for the separation of chemical components from the mixture and for the determination of the structure of the compounds. Analysis plays a vital role in the pharmacy. There are various methods for separation, identification and purification of chemical components by using Chromatography method, High performance liquid chromatography, Gas chromatography, Liquid chromatography, UV spectrometry, Mass Spectrometry.
-
Spectroscopic Techniques
-
Titration Methods
-
Quality Control
-
Chromatographic Techniques
-
UV Spectrometry
Track 10: Quality Assurance & Quality Control
Quality assurance is to prevent defects in deliverables in the planning process itself to avoid the rework, which cost a lot. Quality assurance is a proactive process, and it starts at the early stage of the project to understand the product stated and non-stated requirement and expectations, and then develop the plan to meet these requirements and expectations. Quality control is concerned with the operational activites and techniques that are used to fulfill the requirements of quality. The quality control process includes the activities focus on identifying defects in the actual product being produced.
-
Good Manufacturing Practice
-
Analytical Techniques
-
Sample handlings
-
Calibration Procedures
-
Sampling and Analysis plan
Track 11: Pharmacokinetics & Pharmacodynamics
Pharmacokinetics is study of (ADME) Absorption, Distribution, Metabolism and Excretion of a drug, and pharmacodynamics is the study of relationship between the drug and its receptors, its mechanism of action and therapeutic effect. Both can play a role in drug–drug interactions. Many drugs share the same metabolic pathways or target the same receptors. This leads to the occurrence of pharmacokinetic and pharmacodynamic differences, respectively. Drug–drug interactions occur mainly during drug absorption, distribution (plasma drug binding protein), biotransformation and excretion.
-
Thereapeutic window
-
Area Under Curve
-
Biopharmaceuticals Process Validation
-
Drug Metabolism
-
Pka and Pkd Models
Track 12: Pharmacology & Toxicology
Pharmacology is the science of drugs, it deals with the interaction of externally administered chemical entity (drugs) with living systems. It encircles all aspects of knowledge about drugs, but most essentially those that are relevant to effective and safe use for medicinal purposes. Toxicology is the study of the poisonous effect of drugs and other chemicals (household, the environmental pollutant, industrial, agricultural, homicidal) with emphasis on detection, prevention, and treatment of poisonings. It also includes the study of adverse effects of drugs, since the same substance can be a drug or a poison, depending on the dose.
-
Neuro pharmacology
-
Pharmacognosy
-
Clinical toxicology
-
Receptors
-
Adverse Drug Reactions
Track 13: Drug Targeting and Drug Development
Target drug delivery system is a special form of drug delivery system where the pharmacologically active agent or medicament is selectively targeted or delivered only to its site of action or absorption and not to the non-target organs or tissues or cells. Targeted drug delivery implies for selective and effective localization of pharmacologically active moiety at pre identified (pre-selected) target in therapeutic concentration, while restricting its access to non-target normal cellular linings, thus minimizing toxic effects and maximizing therapeutic index. Targeting of drugs to special cells and tissues of the body without their becoming a part of systemic circulation is a very novel idea. A drug can be administered in a form such that it reaches the receptor sites in sufficient concentration without disturbing the extraneous tissue cells.
-
Passive Targeting
-
Inverse Targeting
-
Ligand Mediated Targeting
-
Liposomes
-
Nano Tubes
Track 14: Biopharmaceutics
Biopharmaceutics is the study of the physical and chemical properties of a drug and its dosage form in which the drug is given, and the route of administration on the rate and extent of systemic drug absorption. Biopharmaceutics is an integral component of the overall drug development cycle. The vitality of the drug substance and the medication regardless detailing ahead absorption, Furthermore in vivo conveyance of the drug of the site about action, will be portrayed as an arrangement for occasions that precede elicitation of a drug’s restorative impact.
-
Therapeutic drug monitoring (TDM)
-
Toxicokinetics
-
Drug concentrations
-
Invivo-invitro protein binding
-
Drug metabolism
-
Protein half life
-
Physical Pharmacy: Physical–Chemical Principles
Track 15: Pharmacovigilance
Pharmacovigilance is “the science and activities relating to the assessment, understanding, detection, and prevention of adverse effects or any other drug-related problems”. Pharmacovigilance is the process and science of controlling the safety of medicines and taking action to decrease risks and increase benefits from medicines. It provides complete and clear information related to drug safety and various risks and benefits associated with them. Pharmacovigilance can help in providing information of unintended and severe adverse events which could not be provided by clinical trials involving in-vivo method. It increases the impact of pharmacovigilance on patient welfare and public health and to know what is pharmacovigilance.
-
Detection And Evaluation Of Drug Safety
-
Pharmacovigilance Legislation
-
Pharmacovigilance And Healthcare System
-
Methods Of Pharmacovigilance
Track 16: Regulatory Affairs
Regulatory Affairs a blend of science and administration to achieve an important objective within a drug development organization. Everything relating to drugs from the earliest non-clinical studies, through development, into routine manufacture and marketing. It can add compelling impact for patients and drug companies. The role of regulatory affairs is to establish and execute a regulatory strategy to ensure that the collective efforts of the drug development team results in a product that is approvable by global regulators but is also differentiated from the competition in some way and also is to ensure that the company’s activities, from non-clinical research through to advertising and promotion, are conducted in accordance with the regulations and guidelines established by regulatory authorities.
-
Good Manufacturing Practice
-
Regulatory Compliance
-
Drug Labelling Approvals
-
Drug Marketing Approvals
-
Manufacturing And Regulatory Compliance
Track 17: Pharmacy Practice
Pharmacy Practice acknowledge to the needs and wants of the people who use the pharmacists services to provide the best or most favourable, evidence-based care. The pharmacists concentrate on effective medicines management and aim to optimise the ease that patients acquire from their prescribed treatment as well as the supply of medicines.
The Pharmacy Practice is to provide medications and other health care products and services and to help people and society to make the best of them. The scope of pharmacy practice includes synthesizing and dispensing medications, modern services related to health care, which includes clinical services, considering medications for safety and efficacy, and furnishing drug information. Pharmacists practice includes community pharmacies, hospitals, clinics, skilled nursing facility, psychiatric hospitals, and regulatory agencies.
-
Pharmaceutical Care
-
Drug Information
-
Prescription and Incompatibilities
-
Future Pharmacy Practice
-
Patient Counselling
-
Pharmacy Practice Research
-
Transcribing Dispensing Administration
-
Hospital Pharmacy
-
Community Pharmacy
Pharmaceutical Market - Global
Pharmaceuticals are one of the world's most beneficial ventures.In 2008 alone, the pharmaceutical business sold $773 billion in items around the world a number that has reliably developed for as long as 8 years and is anticipated to increment again by 2.5 to 3.5 percent in 2017, as per the medication statistical surveying firm IMS Health.
In any case, the procedure that transforms look into dollars into pharmaceuticals is a moderate and regularly strenuous one. It now takes a normal of 12 to 15 years and up to $1.7 billion for a medication to go from revelation to advertise, as indicated by The Pharmaceutical Research and Manufacturers of America. Also, regardless of constantly and cash contributed, just a modest bunch of medications are affirmed by the FDA every year.
The laborious medication endorsement prepare uncovers a focal truth about Big Pharma: it's a standout amongst the most seriously controlled businesses on the planet. The U.S. Nourishment and Drug Administration (FDA) and its European Union partner, the European Medicines Agency (EMEA), represent each part of a medication's improvement from chemicals utilized as a part of the medication and clinical study directions, called conventions, to bundling segments and showcasing materials. This strict oversight is intended to ensure persistent wellbeing, and pharma organizations consider administrative oversight important. The consistent weight to hold fast to government commands shapes each part of a pharma company's association, operations, and culture. For instance, sedate organizations keep up capable administrative undertakings divisions-the offices that arrangement with government offices and they have a tendency to be hazard disinclined.
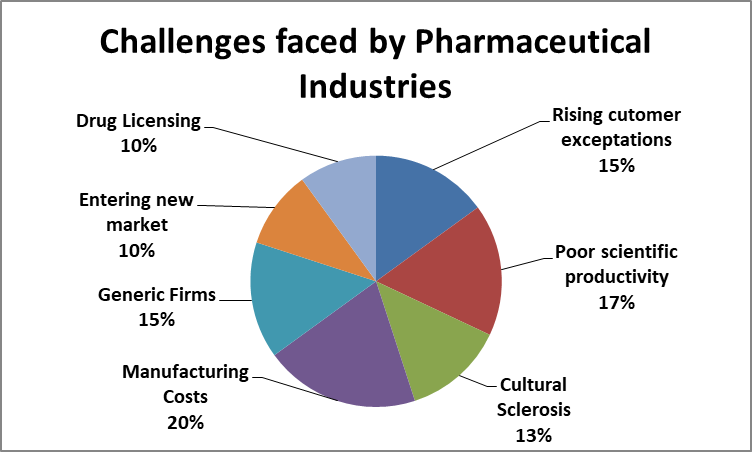
The Pharmaceutical industry's long successful strategy of placing big bets on a few molecules, promoting them heavily and turning them into blockbusters worked well for many years, but its R&D productivity has now plummeted and the environment’s changing. There are few trends which are reshaping the marketplace.
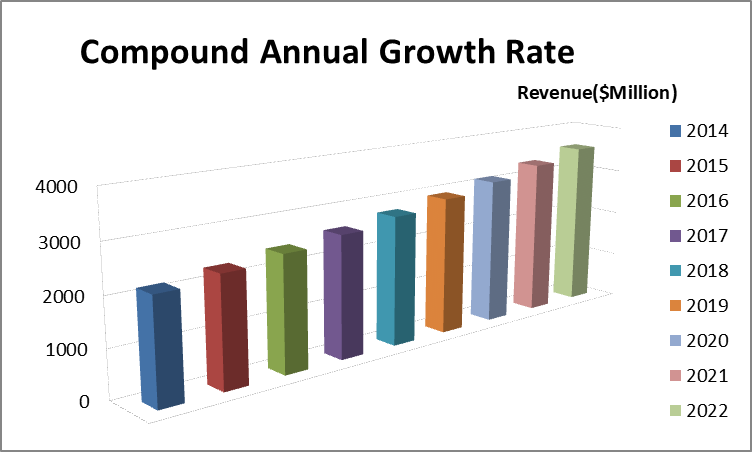
Pharmaceuticals represented a US$300 bn-a-year market globally as of 2015, the World Health Organization states. The global pharmaceutical market is expected to surpass US$400 bn by 2018. Worldwide prescription drug sales forecasted to grow at a robust 6.5% (CAGR) through 2022 to reach $1.06trn . 32% of the 2022 increase in sales to come from orphan drugs (+$95bn).
Pharmaceutical Market - Middle East
The pharmaceutical and healthcare sectors in the Middle East and North Africa (MENA) region will be shaped by numerous factors in 2017, including a slowdown in public sector growth as a result of low oil prices, regional challenges on the back of heightened pressure on drug prices and economic instability. We forecast MENA's pharmaceutical expenditure to increase to USD33.4bn in 2017, up from USD32.2bn in 2016. We also highlight two additional themes that will define the pharmaceutical and healthcare markets of MENA in 2017.
The pharmaceutical and healthcare sectors in the Middle East region will be shaped by numerous factors in 2017, including a slowdown in public sector growth as a result of low oil prices, regional challenges on the back of heightened pressure on drug prices and economic instability. The forecast of pharmaceutical expenditure to increase to USD33.4bn in 2017, up from USD32.2bn in 2016.
Pharmaceutical Market - United Arab Emirates (UAE)
The pharmaceutical market in the United Arab Emirates (UAE) is set to grow from just over $3 billion in 2015 to $5.7 billion by 2020, driven by increased healthcare expenditure, mandatory health insurance, and growing medical tourism.
According to research, UAE is quickly gaining popularity as a medical tourism destination due to its low costs, English-speaking medical staff, and virtually non-existent queues for treatment. Indeed, the Dubai Health Authority is working to develop the medical tourism sector, aiming to attract 1.3 million medical tourists annually by 2021. The revenue on medical tourism is expected to grow by 13% annually for the next five years.
Top universities in UAE:
-
Gulf Medical University
-
Dubai Pharmacy College
-
University of Sharjah
-
Al Ain University of Science and Technology
-
Ras al-Khaimah Medical and Health Sciences University
-
Ajman University
Related Conferences:
1. Annual Congress on Drug Formulation & Analytical Techniques September 03 - 04, 2018 Dubai, UAE
2. Annual Congress on Chemistry in Drug Discovery & Designing April 16-17, 2018 Dubai, UAE
3. World Congress on Bioavailability & Bioequivalence April 16-18, 2018 Dubai, UAE
4. World Congress on Bio-organic and Medicinal Chemistry July 19-21, 2018 Dubai, UAE
5. International Pharmacodynamics and Pharmacotherapeutics Conference October 18-19, 2018 Dubai, UAE
6. International Conference and Exhibition on Pharmacovigilance & Drug Safety June 21-22, 2018 London, UK
7. International Conference on Biopharma and Biotherapeutics October 24-25, 2018 Boston, Massachusetts, USA
8. World Congress on Pharmacology & Therapeutics September 17-18, 2018 | San Antonio, Texas, USA
9. International Conference and Expo on Drug Discovery, Designing & Development September 06-07, 2018 London, UK
10. International Conference on Pharmacy and Pharmaceutical Sciences February 15 - 16, 2018 London, United Kingdom
11. International Conference on Nanopharmaceutics and Advanced Drug Delivery August 16-17, 2018, Dublin, Ireland
12. Annual International Conference on Pharmaceutical Sciences 7th – 8th May 2018 Athens, Greece
13. International Conference and Exhibition on Pharmaceutical Sciences & Research Dubai, UAE, June 8-30, 2018
14. World Congress of Pharmacy and Pharmaceutical Sciences 2 - 6 September 2018, Glasgow, UK
15. International Conference On Pharmacology, June 18-20, 2018 Tokyo, Japan
16. International Conference on Medicinal Chemistry, July 4-6, 2018, Strasbourg, France
17. World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry February 26- 27, 2018 London, UK
18. International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems March 19-21, 2018 Berlin, Germany
19. International Conference and Exhibition on Pharmaceutical Development and Technology May 11-12, 2018 Tokyo, Japan
20. International Conference and Exhibition on Pharmacovigilance & Drug Safety June 21-22, 2018 London, UK
21. Annual European Pharma Congress May 07-09, 2018 Frankfurt, Germany
22. International Conference and Exhibition on Pharmaceutical Nanotechnology and Nanomedicine April 18-19, 2018 Las Vegas, Nevada, USA
23. International Conference on Analytical and Bio analytical Techniques October 31- November 01, 2018 Columbus, Ohio, USA
24. International Conference on Pharmaceutical Formulations & Drug Delivery September 17-18, 2018 | Philadelphia, Pennsylvania, USA
25. International Conference on Clinical Trials September 24-26, 2018 | Chicago, Illinois, USA
26. World Congress on Pharmaceutical Sciences September 28-29, 2018 | Montreal, Canada
27. Annual Medicinal & Pharmaceutical Sciences Congress July 05-06, 2018 Bangkok, Thailand
28. Global Pharmacovigilance & Clinical Trials Summit July 16-17, 2018 Sydney, Australia
29. World Congress on Advanced Pharmacy and Clinical Research July 16-17, 2018 Sydney, Australia
30. International Conference and Exhibition on Pharmacology and Ethnopharmacology July 18-19, 2018 Atlanta, Georgia, USA
Related Societies:
1. Drug Information Association; Pharmaceutical Association of Malaysia
2. Pharmaceutical Group of the European Union; Regulatory Affairs Professionals Society
3. Chinese American Biopharmaceutical Society
4. Parenteral Drug Association
5. International Society for Pharmaceutical Engineering
6. Association of Clinical Research Organizations
7. Pharmaceutical and Novel Drug Delivery Healthcare Sciences Society
8. International Academy of Compounding Pharmacists
9. European Pharmaceutical Union
10. American Society for Pharmacology and Experimental Therapeutics
11. Association of Clinical Research Organizations
12. American Association of Pharmaceutical Scientists (AAPS)
13. US Food and Drug Administration (FDA)
14. American Society for Clinical Laboratory Science (ASCLS)
15. National Cancer Institute Center for Cancer Research (NCI)
16. American Association for Clinical Chemistry (AACC)
17. American Society for Pharmacology and Experimental Therapeutics (ASPET)
18. Association of Clinical Research Organizations (ACRO)
19. International Pharmaceutical Manufacturers Group (IPMG)
20. American Association of Colleges of Pharmacy (AACP),
21. American Pharmacists Association(APhA),
22. American Society for Pharmacy Law American Society of Consultant Pharmacists (ASCP),
23. American Society of Health-System Pharmacists (ASHP),
24. Professional Compounding Centers of America.
25. European Association of Employed Community Pharmacists in Europe (EPhEU),
26. European Pharmaceutical Union (EPU)
27. Controlled Release Society (CRS)
28. Institute of Pharmacy Management International (IPMI)
29. International Academy of Compounding Pharmacists (IACP)
30. International Federation of Pharmaceutical Manufacturers Associations (IFPMA)
ME Conferences conducted the eleventh World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry at Hotel Hyatt Place Amsterdam, Netherlands amid February 27-28, 2017.The conference was composed based on "Most recent Trends in Pharmacy: Spanning the Gap in Research and Product Commercialization". Besides, the systems administration sessions established the framework for quite a while commendable coordinated efforts between many start-up and enormous ventures.
Pharmaceutical Sciences 2017 saw an amalgamation of unbeatable speakers who illuminated the group with their insight and confabulated on different novel subjects identified with the field of Pharma Industry and Pharma Sciences. This congress not just presented the most recent improvements in the field yet additionally gave answers for the various difficulties experienced in building up a Pharma Industry.
Meeting Series might want to pass on a warm appreciation to all the Honorable visitors, Keynote Speakers, Delegates, Media Partners and Exhibitors for their investment in Pharmaceutical Sciences 2017.
The conference was initiated with the Honorable presence of the Keynote forum. The list includes:
Christopher Tirotta, Director Cardiac Anesthesia, USA.
Geoffroy Lumay, Associate professor - Soft Matter Physics chair, Belgium.
Tirasak Pasharawipas, Professor in Medical Science Department, Thailand.
Pierre A. Morgon MRGN Advisors, Switzerland.
Harish Rajak, Assistant Professor of Pharmaceutical Medicinal Chemistry, India.
Pillhun SonProjects Coordination Manager at CAS a division of the American Chemical Society, USA.
Identification of an enzyme caused vascular damage by due to aircraft noise
From a current study, an enzyme has been identified by scientists which caused vascular damage due to aircraft noise. Researchers also said that the enzyme caused vascular damage at night-time noise and the night-time sleep should be protected from noise.
Aircraft noise caused an increased development of cardiovascular diseases. Researchers found a disease called endothelial dysfunction caused by simulated nocturnal noise. In this disease some disorders are shown like increased the stress hormone epinephrine, reduces sleep quality, and damages the vascular system.
Aircraft noise also effects the brain, the scientists examined. The scientists put their focus on neuronal nitric oxide (NO) synthase, responsible for learning and memory. Due to aircraft noise, the enzyme cannot work properly and the function is damaged.
The scientists also found that the transcription factor FoxO3 helps in noise-induced vascular and brain damage. That was observed that the down-regulation of this transcription factor is done by night-time noise. For this, a defective gene expression network is activated which controls cellular events which is a function of circadian rhythm. If the circadian rhythm is disturbed, sleep disorders, more cardiovascular, mental, and metabolic disorders was found. The vascular damage will be prevented by treatment with the FoxO3 activator Bepridil.
Identification of brain metal for Alzheimer’s disease
A research team has identified and characterised brain metals by which the progression of Alzheimer’s disease may be precipitated.
The revelation is relied upon to help in understanding the Alzheimer's illness, which causes dementia and memory misfortune, and encourage the advancement of successful treatments in future.
The group effectively portrayed iron species in the mind that causes the arrangement of amyloid protein plaques. These plaques bring about poisonous quality in the cerebrum and prompts cell and tissue demise.
Various kinds of synthetically diminished iron species, including a sort of attractive iron oxide called magnetite, are framed in the amyloid protein plaques. These parts are not regularly found in a solid human mind.
The group used propelled estimation capacities to show point by point confirm that these procedures happen in the brains of patients influenced with Alzheimer's illness.
In the amyloid plagues, researchers also observed the presence of forms of calcium minerals.
The exploration was directed by obtaining amyloid plaque centers from two expired patients who were determined to have Alzheimer's sickness.
Metabolomics
Metabolomics is the all-encompassing investigation of digestion. The use of metabolomics in pharmacology offers ascend to another field called "pharmacometabolomics." The essential principle of pharmacometabolomics is to decide the impacts of medication treatment on the body's metabolic situation. It is likewise used to distinguish particular metabolic pathways that are in charge of medication interceded results. Some interrelated fields, like pharmacology, clinical pharmacology, drug discovery, clinical trials, and personalised drug treatment may be enclosed by the pharmacometabolomics.
Importance: The pathogenesis of human infection is a perplexing procedure that is related with the breaking down of cell frameworks going from genome to digestion. An unfavourable blend of ailment heterogeneity and differential medication reaction phenotypes among people makes the circumstance more mind boggling regarding building up a specific medication treatment. Basically, the pathogenesis of a specific malady may fluctuate among people; comparably, a person's reaction to a specific medication treatment isn't generally the same. Since the hereditary cosmetics of an individual fundamentally impacts ailment pathogenesis and related medication reaction, the significance of genomics can't be overlooked at any cost; nonetheless, with the headway of logical information, it is currently evident that hereditary foundation isn't the sole supporter in the field of clinical science.A critical part of impact additionally originates from natural elements, age, sexual orientation, ailment subtypes, and gut microorganisms. Therefore, tending to the impacts of every one of these variables is of prime significance keeping in mind the end goal to create viable remedial mediations. Pharmacometabolomics is assigned to meet this prerequisite, as every one of these variables has critical impacts on digestion.
Application: Pharmacometabolomics plays important roles in the following fields:
1. Individual drug-response prediction: Pharmacometabolomics goes about as a helpful apparatus in foreseeing singular medication reaction. The methodology starts by making a populace based medication reaction bend against a specific infection utilizing tests gathered when the medication treatment. A short time later, metabolic profiling is performed on tests which are chosen from the closures of the bend (high responders or low responders) or over the bend (general responders).
2.Evaluation of drug-response variability: The interchange amongst pharmacogenomics and pharmacometabolomics is a critical factor. It is also significant in particular drug-response phenotype of a particular medical situation. A specific drug treatment through pharamcometabolimics mat affected the selction of metabolic pathways. This could likewise give important understanding about the systems engaged with tranquilize reaction changeability.
3. Identification of biomarkers: Pharmacometabolomics assumes an imperative part in recognizing biomarkers which is a fundamental advance towards creating analytic apparatuses. Profiling of medication prompted changes in metabolites or making metabolite marks of various malady subtypes, (for example, intrusive or non-obtrusive tumors) through high determination focused on metabolomics devices fills a critical need of biomarker disclosure and symptomatic instrument improvement.
4.Drug discovery and development: The cell destiny of medications regarding assimilation, statement, digestion, and disposal is fundamental in assessing drug viability and lethality. Pharmacometabolomics gives a straightforward and speedier method for portraying drug digestion and pharmacology and also their symptoms. This procedure encourages medicate revelation and improvement.
New approaches to Chemotherapy
In the world, about half of all drugs, ranging from morphine to penicillin or naturally derived drugs include many cancer drugs. They are very toxic and have the power to kill cancer cells.
Researchers have revealed a formerly obscure component - proteins that cells use to tie to a harmful substance and sequester it from whatever is left of the life form.
The work has imperative ramifications for seeing how human growth cells create protection from normal item based chemotherapies. Besides, the microbiome may assume a part in sedate obstruction.
Natural products, chemical compounds produced by living organisms are considered one of the best sources of new drugs and drug leads. Characteristic items may originate from blooms, trees or marine creatures, for example, wipes. A standout amongst the most well-known sources, be that as it may, is soil-abiding microorganisms.
The scientists centered on a class of common items called enediynes. These mixes originate from microorganisms called actinomycetes, which are normally found in the dirt. Two enediyne items are as of now FDA affirmed as malignancy medicates and are in wide utilize. Be that as it may, patients who take them frequently create opposition. Following a time of months or years, tumors can quit reacting to the chemotherapy and start developing once more.
While how patients create protection from these medications remains to a great extent obscure, researchers have revealed two instruments that microscopic organisms use to shield themselves from enediynes.
In the new examination, specialists report a third, beforehand unfamiliar obstruction instrument. It includes three qualities called tnmS1, tnmS2, and tnmS3, which encode proteins that enable microscopic organisms to oppose the impacts of a sort of enediynes called tiancimycins. Scientists are of now examining tiancimycins, which hold incredible guarantee for new Tumor drugs. The proteins work by authoritative to tiancimycins and keeping them isolate from whatever is left of the living being.
In the wake of finding these qualities in actinomycetes and how they function, the specialists considered how across the board these qualities are in different microorganisms. They were shocked to find that notwithstanding actinomycetes, the qualities were likewise present in a few microorganisms normally found in the human macrobiotic, the accumulation of microorganisms that normally possess the human body.
New discovery of a drug to inhibit a vital metabolic process essential for cancer cells’ growth and survival
Therapeutic Discovery team developed the first small molecule drug, IACS-10759, from the concept to clinical trial including IACS and the CCCT. Therapeutic Discovery is a group of clinicians, researchers and drug development experts which are unique in their work.
An emerging hallmark of tumor biology known as metabolic reprogramming where cancer cells developed on two metabolic processes, glycolysis and oxidative phosphorylation (OXPHOS), for their growth and survival. Extensive efforts have focused on therapeutic targeting of glycolysis which stimulated the discovery and development of IACS-10759 which is a potent and selective inhibitor of OXPHOS. This clinical trial was made possible by more than 25 scientists across Therapeutics Discovery.In pre-clinical studies, IACS-10759 was calculated for treating lung cancers in the SMARCA4 gene which provide tumors sensitive to the drug. SMARCA4 is a component of SWI/SNF, a chromatin modeling complex, plays an essential role in gene expression.
New discovery to identify antibiotics
Scientists summarize new methods of fragment-based lead discovery (FBLD) to identify new compounds as potential antibiotics.
Scientists clarify how FBLD works and delineate its focal points over ordinary high-throughput screening (HTS). In particular, how FBLD expands the odds of discovering hit mixes; how its techniques can convey hits without the enormous speculation required for HTS; and how by beginning little, FBLD gives therapeutic scientific experts more chances to construct more medication like mixes. These standards are shown in the survey and upheld with on-going cases of disclosure ventures against a scope of potential anti-microbial targets.
The ascent in anti-infection opposition is presently perceived as a genuine danger to human wellbeing. Be that as it may, no new anti-toxins have been created in numerous decades. FBLD starts by distinguishing low sub-atomic weight mixes (sections), which tie to protein targets. Data on how the pieces tie to their protein targets is then used to develop the mixes into powerful medication hopefuls. Since the pieces are little, they will probably fit into a coupling site and each section speaks to a colossal number of potential mixes.
New discovery to verify the safety of live vaccines without using animal
Scientists discovered another method used to verify the safety of vaccines like yellow fever vaccine without animal test. This is mainly done by an in cellulo device using a 3D culture model known as “BBB-Minibrain”, for evaluating the safety of live vaccines which use for human.
Many years ago, the practice of animal testing by the scientists are hindered by a lack of proper alternatives, particularly in regulatory testing for live viral vaccines, such as yellow fever vaccines. Currently these tests are done on animals, by which clinical signs in the central nervous system are shown and suggest neurotoxic adverse effects.
The “BBB-Minibrain” formed of a blood-brain barrier connected with astrocytes and microglia (a minibrain). It is used to detect the entrance of viruses in the brain through BBB and any neurotoxic effects.
Scientists doing work continuously to develop this test. The BBB-Minibrain model increases the belief to establish a superb method that can be used in pharmaceutical industry to perform the regulatory tests on live viral vaccines.
A new strategy for treating Crimean Congo hemorrhagic fever
In a viral hemorrhagic sickness where up to 40 percent of individuals creating it pass on, it is momentous that specialists still don't concur whether the main perceived treatment, an antiviral medication called ribavirin, has any kind of effect.
Crimean Congo haemorrhagic fever is spread by the chomp of a tainted tick, and is ending up more typical, with episodes in Turkey, Eastern Europe and the Eastern Mediterranean. Specialists treat the disease in doctor's facility with intravenous liquids, blood and great nursing care. The open deliberations around ribavirin are basic among clinicians treating the illness, with solid promoters on one side, and other people who have arrangements not to utilize it, so the creators trusted the survey would settle the level headed discussion.
The survey creators discovered only one preliminary with 136 members, and some observational relative investigations of 612 members: in general the examination did not give an unmistakable answer. At the point when the creators analysed investigations that were regularly cited as demonstrating advantage, they were fundamentally one-sided. Albeit less individuals kicked the bucket in bunches accepting ribavirin, the clear impact could be because of the medication, or similarly in light of the fact that those getting the medication may have likewise been less debilitated, or gotten excellent nursing and therapeutic care prior in the malady.
A few specialists advocate giving ribavirin, and express that not to give it is even deceptive. The issue is that the investigations guaranteeing to exhibit profit by the medication are outlined in such a way they can't separate the impact of the medication from different components, and along these lines they don't know whether ribavirin is powerful by any stretch of the imagination.
The audit illuminates the requirement for dependable research from a randomized control preliminary to set up whether ribavirin is powerful. Specialists have to know whether the medicine works, when it works, and how useful it is.
While inquire about into rising irresistible sicknesses and amid episodes is troublesome, the group trusts that the survey gives a chance to fortify the call for more noteworthy strides to be taken to encourage thorough research giving solid outcomes in flare-ups of irresistible infections.
Non-opioid Drugs Relives Pain in Mice
Specialists have discovered that hindering a receptor on insusceptible cells called macrophages may help relieve pain in a few patients, especially those with endless neuropathic torment, for example, those with conditions, for example, diabetic neuropathy. Looked with the plague of opioid compulsion, analysts have been accused of finding different methodologies to treat torment. Their endeavors to a great extent have concentrated on nerve cells that transmit torment signs to the spinal rope and mind. Yet, new research, demonstrates that focusing on receptors on resistant cells might be more successful, especially for perpetual agony. As of late, a non-opioid, investigational sedate called EMA401 has demonstrated guarantee as a treatment for waiting nerve torment following shingles contamination. While attempting to see how that medication helped control torment, the Washington University investigate group was amazed to find that it doesn't hit nerve cells; rather, it focuses on a receptor on resistant cells.
The investigational tranquilize restrains the angiotensin II compose 2 receptor that is focused by meds that lower pulse. Angiotensin is a hormone that causes veins to tighten, expanding circulatory strain. This medication was thought to work by communicating with the sort 2 receptor on nerve cells - similar cells that convey torment signals. In different examinations in which they infused the angiotensin hormone into mice, the creatures showed they felt torment and pulled back their paws when contacted.
At the point when the scientists diminished the quantity of macrophages in mice, the creatures didn't seem to feel torment in light of an angiotensin infusion. Be that as it may, as the macrophages repopulated through the span of a couple of days, the reaction to torment returned. To help these perceptions in mice and the way of life dish, the scientists likewise have discovered expanded quantities of macrophages close by deteriorating nerve filaments in skin biopsies taken from the legs of patients who have diabetic neuropathy. Expanding the quantity of potential focuses for painkillers and including targets, for example, receptors on insusceptible cells may make it conceivable to grow more powerful painkilling drugs with fewer reactions.
Researchers Interface Protein To Seizure Suppression
As indicated by new examination directed by scientists seizures can be supressed in the brain by a protein called T-cell intracellular antigen-1 (TIA-1).
This investigation gave another understanding on epilepsy– a neurological issue in which nerve movement in mind is influenced prompting repetitive, ridiculous seizures.A scientist says that TIA-1 helps in the control of quality articulation amid cell push. His exploration focusses on the concealment of serious electrical tempests of cerebrum which prompt seizures.
They believed that TIA-1 was incorporated into seizure concealment yet the examination discoveries prescribed something uniqueThe examination creators watched the elements of a catalyst in the mind called cyclooxygenase-2 (COX-2). This catalyst produces prostaglandins; synthetic exacerbates that help in the execution of general undertakings, alongside memory and learning.
The present investigation distinguished that seizure concealment was connected with a bring up in COX-2 articulation in neurons. The analysts meant to comprehend the connection between TIA-1 and COX-2. They began their work by arranging, directing and dissecting tests.
A Scientist presented the primary TIA-1's thorough articulation profile in the ordinary mind. Hefound that TIA-1 smothers epileptogenesis – a moderate procedure of ordinary cerebrum to end up epileptic. His exploration planned to comprehend the sub-atomic systems behind epilepsy; especially, the qualities required with seizure improvement and epileptogenesis.
New Findings Could Help Comprehend and Treat Glaucoma
The Future of Pharma-3D Printing
Conveyance Benefit: How Implantable Drug Delivery Gadgets are Helping Persistent Care
Implantable drug eluting devices(also known as implantable drug delivery systems) offer a few preferences over ordinary oral or parenteral medication conveyance strategies. For example, they can give confined, site particular medication delivery, which is particularly imperative in applications, for example, cardiology and oncology, where focused conveyance can enhance the adequacy of treatment and limit reactions or harm to sound tissue.The measurement necessities regularly are lower than options, additionally lessening the potential for side effects.Also, sedate eluting gadgets can enhance understanding consistence, one of the best difficulties in human services, as around half of ordinary drugs are not utilized as prescribed.The treatment regimen can be less complex since it requires less specialist's visits and doses than customary therapies.
Uses of implantable drug eluting devices incorporate, among others, diabetes administration, contraception, HIV/AIDS avoidance, incessant agony administration, cardiology, oncology, and focal sensory system (CNS) wellbeing. Alongside subcutaneous implantation, different body locales can fill in as implantation destinations (e.g. intravaginal, intravascular, intraocular, intrathecal, and peritoneal).
There are two classes of drug-eluting devices: Biodegradable and non-biodegradable. Biodegradable drug-eluting devices (likewise alluded to as bioerodible) utilize biocompatible materials, for example, polyesteramide (PEA) and poly lactic-co-glycolic corrosive (PLGA) to convey drugs, and, once embedded, disintegrate after some time.
Rather than biodegradable, non-biodegradable drug delievery devices(additionally alluded to as biodurable) utilize biocompatible materials like silicone elastic (polydimethylsiloxane or PDMS), polyethylene-vinyl acetic acid derivation (EVA), and thermoplastic polyurethane (TPU) to convey drugs. Non-biodegradable medication eluting gadgets can be planned as grid, store, or osmotic frameworks to convey drugs by means of dispersion or osmosis and are by and large less exorbitant than biodegradable devices. Non-biodegradable medication eluting gadgets can be refilled with solution (e.g. by means of infusion) and the gadget's belongings are very quickly reversible upon expulsion
Non-biodegradable Pathway TPU excipients are intended to be adaptable and customisable to an expansive scope of compound and physical properties giving assortment along various measurements, including drug discharge energy (short or long haul); dynamic pharmaceutical fixing determination (hydrophobic or hydrophilic APIs); handling strategies (expulsion, infusion trim or dissolvable throwing), and mechanical performance. These traits furnish engineers with plan adaptability.
Digital PHARMA
Chemically Modified Drug to Treat HIV
In an enormous breakthrough that would accelerate a possible HIV treatment, a studies team has altered the chemical structure of a present antiviral drug to ease it in reaching cells and tissues where HIV resides.
The use of a physiochemical scheme that alters the properties of the drug dolutegravir, Scientists took the changed drug and positioned it into nanocrystals. The produced drug crystals effortlessly distributed in the course of the body to tissue reservoirs of HIV infection.
The advanced drug scheme prolonged the existence of the drug and its entry into "hidden body compartments," from the muscle site of injection at the same time as growing its action in lowering viral growth. The tissues covered the lymph nodes, the bone marrow, the intestine and the spleen.
The modified drug crystals have been not poisonous, did not destroy apart with temperature modifications and had been stable for months of time. All organs and physical functions remained intact after treatment.
Researcherssays covered with components of fats,thecrystals efficaciously maneuvered via cellular defensivemembranes and have been stored interior cells for weeks.
When stored interior cells known as macrophages, the drug was gradually discharged from the crystal in an altered inactive form known as a "prodrug". The cell after that breaks the prodrug into an active drug, and the active drug is then launched to circulate from the cell and tissue stores.
The power of this system is that it not simplest may be powerful in improving HIV care and prevention. However can be applied to many classes of drugs beyond HIV, including drugs used to deal with cancer, other infectious illnesses and degenerative diseases that have an effect on the brain
The new products can optimize HIV restrictive increase in order that techniques that can eliminate viral infection might achieve success
New Drug Capsule for HIV Treatment
Researchers have
The novel drug is designed therefore the patients can acquire it easily once in a week then the drug will release steadily all through the week. This kind of delivery system couldn't only enhance patients' adherence to their remedy schedule however additionally be utilized by people prone to HIV exposure to help prevent them from turning into infected.
One of the primary limitations to treating and preventing HIV is adherence.
The capacity to make doses much less common stands to enhance adherence and make an extensive effect on the affected person level.
Scientists developed a new drug-
Several clinical trials have evaluated whether antiretroviral pills can prevent HIV infection in healthy populations. Those trials have had blended success, and one main impediment to preventative treatment is the problem in getting people to take the essential pills each day
Researchers believed that a drug delivery pill they developed in may assist to cope with this problem. Their pill includes a star-shaped structure with six arms that can be loaded with tablets, folded inward, and encased in a smooth coating. After the pill is swallowed, the arms unfold and steadily release their cargo.
In their authentic model, the whole star shape was made from one polymer that both gives structural aid and carries the drug payload. This made it harder to design new pills that could release drugs at varying rates, because any changes to the polymer composition may disrupt the pill's structural integrity.
To overcome that, the researchers designed a novel form in which the backbone of the star structure remains a strong polymer, however every of the six arms might be full of a unique drug-loaded polymer. This makes it simpler to design a pill that releases drugs at unique rates.
Nanopharmaceutics
Nanotechnology is a study associated with nanoparticles, their ability and practice regarding unique frameworks. The part of nanotechnology within the discipline of pharmaceutics has outstandingly changed the approach for our comprehension approximately medicinal drugs, Nano pills or the usage of nanoparticles as transporter of medicine has become the critical component or criteria for the introduction or plan of a medication.
Many therapeutic agents offer a low beneficial dose (dose liable for efficacy)/useless dose (dose eliminated or responsible for toxicity) ratio, particularly because of the fact that therapeutic agents ought to ensure in one single item all of the functions required to deliver the remedy, which results in compromises in their physico-chemical layout. Scientists have introduced the concept of priming the body to obtain the remedy with the aid of uncorrelating these capabilities into distinct items sequentially administered: a nanoprimer occupying transiently the primary pathway liable for therapeutic agent restricted benefit/threat ratio observed by way of the healing agent. The idea became evaluated for specific nature of healing dealers: For nanomedicines they designed a liposomal nanoprimer providing preferential hepatic accumulation without signal of acute toxicity. This nanoprimer changed into capable of increase the blood bioavailability of nanomedicine correlated with a decrease hepatic accumulation. Subsequently this nanoprimer markedly superior anti-tumor efficacy of irinotecan loaded liposomes inside the HT-29 tumor model while in comparison to the nanomedicine alone.
Conference Highlights
- Drug Discovery & Development
- Novel Drug Delivery System
- Pharma and Technology
- Pharmacogenomics
- Clinical Research
- Advances in Pharmaceutical Packaging
- Bioavailability & Bioequivalence
- Pharmaceutical Formulations
- Pharmaceutical Analysis
- Quality Assurance & Quality Control
- Pharmacokinetics & Pharmacodynamics
- Pharmacovigilance
- Pharmacology & Toxicology
- Drug Targeting and Drug Development
- Regulatory Affairs
- Pharmacy Practice
- Biopharmaceutics
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | September 24-25, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- esearch & Reviews in Pharmacy and Pharmaceutical Sciences
- esearch & Reviews in Pharmacy and Pharmaceutical Sciences
- esearch & Reviews in Pharmacy and Pharmaceutical Sciences
Abstracts will be provided with Digital Object Identifier by